How can you confirm that your qNMR experiment was performed with completely relaxed protons? By using the Alternative Relaxation Time Determination (T1)
Basics of qNMR
NMR has become a powerful technique to solve analytical problems. Especially the number of quantitative NMR (qNMR) applications has increased over the last two decades [1]. There are only five preconditions to be considered for accurate measurements and evaluations of qNMR experiments: (i) selectivity, (ii) inert substances, (iii) total solubility, (iv) sufficient signal/noise ratio and finally (v) totally relaxed signals [2].
To guarantee fully relaxed signals, a relaxation time determination in front of every quantitative experiment is necessary. Classical T1 measurements run for several minutes or even hours leading to a need for a fast relaxation time determination. An alternative pulse sequence for relaxation time determination is developed and demonstrated in Fig. 1.
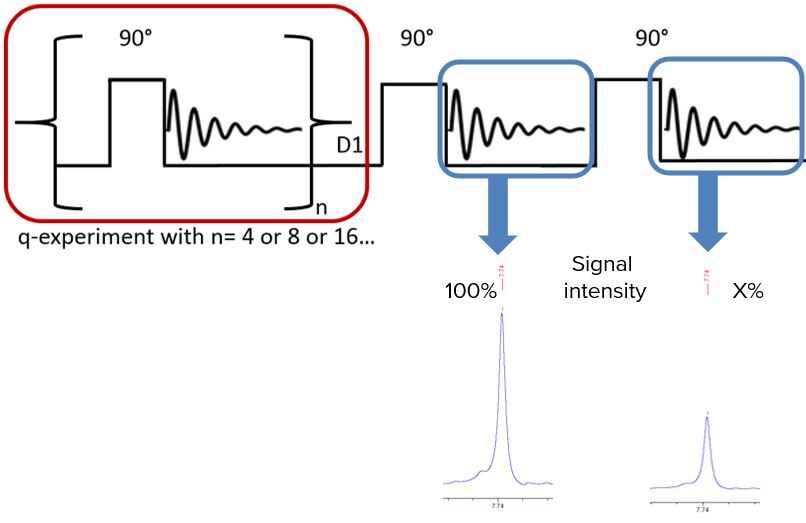
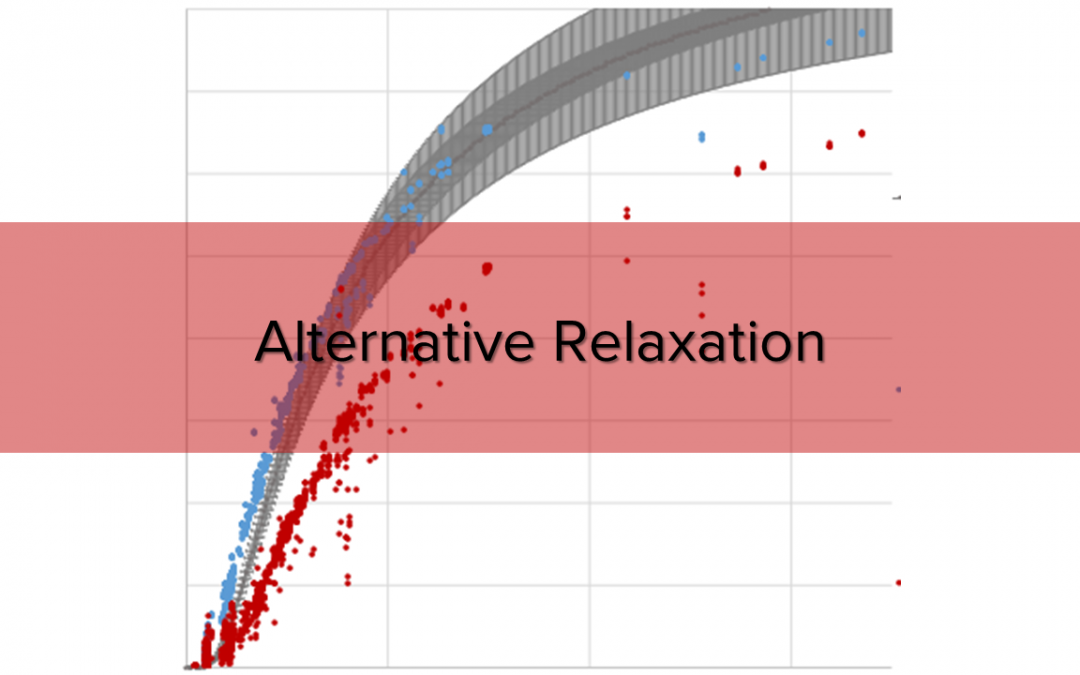
Fig. 1. Alternative pulse sequence for a fast relaxation time determination by comparing totally relaxed signals with partially relaxed signals.
Longitudinal Relaxation Time T1
T1 depends on the effects of anisotropic and segmental motion, the number of attached hydrogens and the dipole-dipole relaxation by oxygen. Hence, how can we know that all protons used for qNMR are totally relaxed without running a full T1 experiment? The solution: two 90° pulses with a specific delay time set up! The experimental set up underlines the fact that recovery of magnetization along the z-axis proceeds after first order kinetics [3].
The first additional 90° pulse is set after a delay time of 60 seconds. For the second one, D1 equals for instance the acquisition time of the FID causing a spectrum with only partially relaxed signals. Comparing these two signals a specific intensity ratio is observed. By knowing this intensity ratio and the delay time prior the second pulse the relaxation time can be calculated by:

Influences on Relaxation times
Parameters like oxygen, solvent, temperature and the composition of a mixture itself have a strong influence on the relaxation times and may change them by a factor of up to 200 % [4]. T1 estimation by checking the chemical structure or having a look at literature values is only applicable to a certain extent.
Using the blue framed pulse sequence from Fig. 1 over 3,200 peaks have been evaluated and collected for Fig. 2 where T1 was measured by inversion recovery. The results of the 180°:90° pulse sequence match perfectly to the theoretical relaxation processes, a very precise T1 calculation is possible. In contrast to this, the pulse sequence (90°:90°) shows a general deviation in height. This deviation may be explainable by taking transversal relaxation processes into account. The sequence never aims at a precise T1 determination, however at a fast and rough one. As a time buffer is always added to the delay time between two subsequent pulses the deviation between calculated and “real” T1 is negligible.
Power of The New Pulse Sequence
Running a qNMR experiment, there is a fundamental need to have knowledge about the relaxation times. The additional 90° pulses give the possibility to calculate roughly the relaxation times of the corresponding H-atoms. In contrast to well-established T1 methods, this pulse sequence expands the qNMR experiment by only two additional pules. Consequently, the total experiment is only expanded by round about one minute including all delay times! Moreover, using this mathematical way, a written evidence for the right D1 set up is given. The guarantee of fully relaxed signals enforces the meaning of qNMR in regulatory working areas such as pharmaceutical industry which strongly demands for GMP and GLP. So is this pulse sequence innovative enough to serve for the fundamental need for a fast and reliable relaxation time estimation?
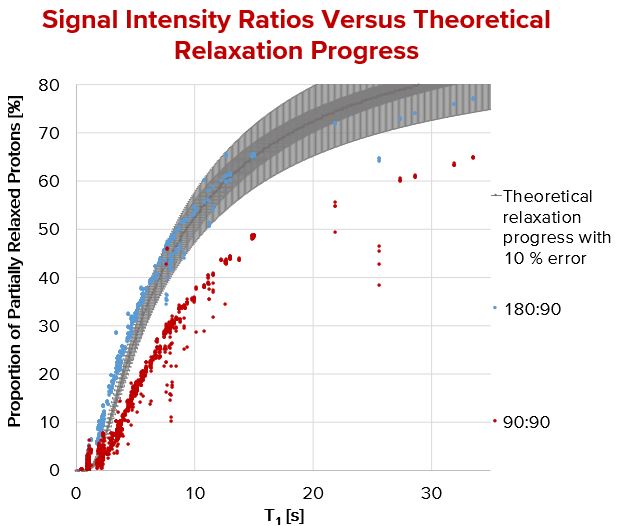
Fig. 2. Calculated signal intensity ratios compared with their relaxation time. T1 was determined by inverse recovery.
References
[1] M. Weber et al., Journal of pharmaceutical and biomedical analysis 2014, 93, 102.
[2] U. Holzgrabe, Progress in nuclear magnetic resonance spectroscopy 2010, 57 (2), 229.
[3] A. B. Kudryavtsev, W. Linert, Physico-chemical Applications of NMR: A Practical Guide, Bd. 1, World Scientific 1996.
[4] N. Nestle, T. Baumann, R. Niessner, Water Research 2003, 37 (14), 3361.
The research of the alternative T1 determination was performed by Patrick Jonas and Prof. Dr. Bernd Diehl