Qualitative analysis
NMR spectroscopy combined with chemometrics was used to distinguish heparin and low molecular weight heparin (LMWH) produced from porcine, bovine and ovine tissues as well as their blends. The models were validated for the modeling of spectral data of more than 200 authentic samples. Moreover, the intra-species differences within the each animal group according to the manufacturer were examined. Significant improvement of chemometric models was achieved by switching to 2D NMR experiments (heteronuclear multiple-quantum correlation (HMQC).
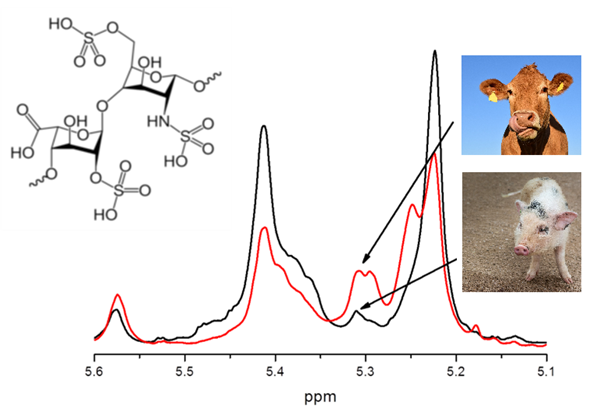
Moreover, retrospective multivariate analysis was performed on a big dataset of 990 NMR routine heparin spectra recorded over six years (2012-2017) in our laboratory. Several steps of statistical analysis of accumulated data were used to differentiate heparin samples according to animal origin (bovine, porcine and ovine), purity grade (crude and purified), distributing company as well as to estimate the closeness of their structure to the heparin reference sample provided by USP (Fig. 1).
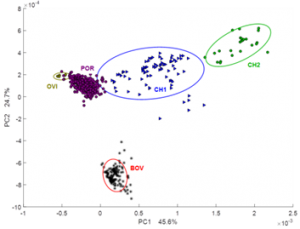
Fig. 1. Scatter plot of the PCA scores values for 870 heparin spectra of different origin (BOV – bovine; POR – porcine; OVI – ovine) and 111 crude porcine heparin (CH1 and CH2). Ellipses show 95% probability for each group.
Quantitative analysis
In addition to the above-mentioned qualitative characteristics, NMR also allows considerable amount of quantitative information to be obtained using the standard USP sample preparation. Our unique holistic approach includes the determination of molecular weight based on DOSY experiment as well as water content, inorganic ions (Na+, Ca2+, Cl–, and CH3COO–) based on the subsequent 1H, 2D, 23Na and 35Cl NMR runs with a total measurement time less than 20 minutes (Fig. 2). Validation results in terms of precision, reproducibility, limit of detection and recovery demonstrated that the developed method is fit-for-purpose for the authentic heparin samples. Semi-quantitative assessment of anticoagulant activity is also feasible.
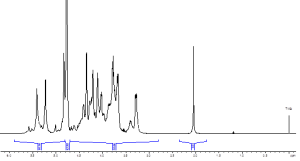
Fig. 2 Water quantification in heparin by 1H NMR spectroscopy
Holistic control with only one sample preparation according to Pharmacopeia using just four sequential NMR experiments combined with chemometrics provides purity, assay and provenience of heparin samples as well as enables correlation with biological activities and macroscopic values.
For details please refer to our constantly growing list of scientific publications:
Monakhova YB, Diehl BWK. Nuclear magnetic resonance spectroscopy as a tool for the quantitative analysis of water and ions in pharmaceuticals: example of heparin J Pharm Biomed Anal. 2018, accepted
Monakhova YB, Fareed J., Yao Y., Diehl BWK, Improving reliability of chemometric models for authentication of species origin of heparin by switching from 1D to 2D NMR experiments J Pharm Biomed Anal. 2018, accepted