NMR spectroscopy is a powerful tool for the quality control of vaccines based on mRNA and peptides under GMP!

Challenges of the current situation
The worldwide efforts to address the global COVID-19 pandemic led to numerous projects of scientific research, clinical trial and release to the market. Thus, increasing the examination demand of developed vaccine products. Nuclear magnetic resonance spectroscopy (NMR) as a strong analytical tool offers several approaches to be of help in the testing of used raw materials, intermediates, solvents, impurities and release of final product batches.
mRNA investigation by NMR
mRNA becomes an important topic in pharmaceutical products such as vaccines. Therefore, NMR spectroscopy is a powerful technique for a holistic quality control. For distinguishing single building blocks of RNA and also including modified nucleosides, 1H-NMR spectroscopy proves to be optimal (see Fig. 2). Furthermore, the content of RNA and its degradation products like free phosphate or pyrophosphate are determined quantitatively by 31P-NMR spectroscopy. Sodium as counterion is examined by 23Na-NMR spectroscopy to confirm the correct stoichiometry. This enables a powerful opportunity for all steps of manufacturing pharmaceuticals such as vaccines to be monitored by NMR for quality control under GMP.
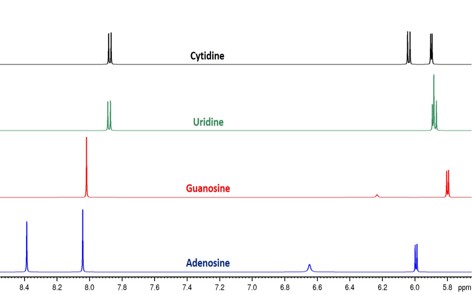
Fig. 2: 1H-NMR spectra overlay of standard nucleosides of mRNA with distinctive signals of the nucleobase and the sugar proton at C1‘ between 5.5-8.5 ppm.
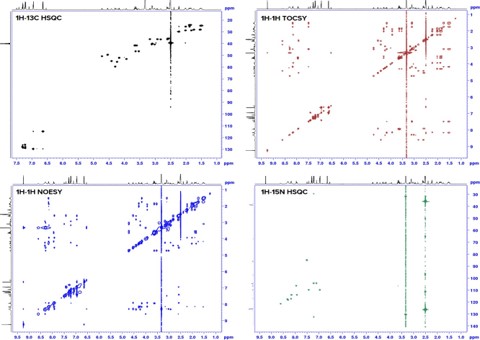
Fig. 3: 2D-NMR spectra offer a variety of information about peptides molecules using through-bond (black, red, green) and through-space (blue) correlation methods.
Peptide determination by NMR
Besides determination of purity by qNMR, NMR spectroscopy is the technique of choice when structure elucidation is required. Due to the atom selective nature of the NMR measurement specific bonds within the molecule and their respective through-bond magnetic correlation are targeted. Consequently, NMR spectroscopy provides information about the primary, secondary and tertiary structure of peptides.
As a GMP/GLP-certified, FDA approved and independent testing laboratory, we are pleased to analyze your pharmaceutical products according to GxP standards and with great expertise in state-of-the-art NMR spectroscopy.