Manganometry have been used for the determination of the quality of water for over 50 years. The water contains an unknown concentration of oxidizable substances such as amino acids or carbohydrates which can be titrated by using a strong oxidizing agent, here potassium permanganate. Permanganate (MnO4–) can be easily reduced to different oxidation states of the manganese depending on the pH value. In an acidic environment permanganate is reduced to Mn(II) whilst in neutral and alkaline solutions it yields in Mn(IV) ions. Here the advantage of the great violet color of potassium permanganate is taken (Fig. 1).
Fig. 1 Pure potassium permanganate

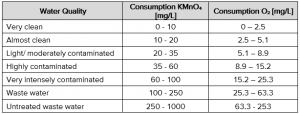
During the titration and thus the reduction, the strong violet color of the potassium permanganate disappears with the concentration of the oxidizable substances and the reaction can be identified visually. Since the solution achieved a pink coloration which has not been changing for 30 seconds, a sufficient saturation is reached. The volume of the potassium permanganate can be easily recalculated into the amount of reacted potassium permanganate, and thus the potassium permanganate index and the amount of oxygen in the sample (equation 1).The amount of reacted potassium permanganate is used to assess the water quality status based on Tab. 1.
Equation 1: Calculation of reacted potassium permanganate amount
![]()
Tab. 1 Assessment of water quality (HTKW Leipzig, 2017)
Water quality is not equal water quality
The quality of the water in food production must meet the standards governing the quality of drinking water as set out in Council Directive 98/83/EC and implemented in Ireland under the European Union Drinking Water Regulations, 2014 (S.I. No. 122 of 2014). Here, all parameters are set for water as a food product. But how can we determine the water quality in water as an aquatic vegetation? Here, temperature, pH, total phosphorus and nitrate nitrogen are important as physical and chemical parameters. Furthermore, the animal, fish and algal species composition is a meaningful parameter for the biology of waters. For us as analytical chemists, the chemical point of view is interesting. This was the reason why we examined the reaction of several water samples with potassium permanganate.
An alternative 55Mn NMR method
The titration method is very time-consuming requiring a high volume of chemicals and solvents. To simplify the analysis of oxidizable substances in water, an NMR spectroscopy method was developed – and what makes it so special is that we run 55Mn NMR spectroscopy! At first, we analyzed the prepared potassium permanganate solution in D2O as a blank value. The integration value of the 55Mn NMR signal gave us a starting value where no oxidizable substances were present. Then, Eike was biking around Cologne to collect several water samples from the following lakes and rivers: Volksgarten, Rhine, Otto-Maigler lake, Aachener pond, waste water before and after cleaning, tap water at Spectral Service and the Uni-Center, deionized water and D2O. Each water sample was mixed with the potassium permanganate solution which was present in in excess, incubated for one hour at 60°C and afterwards analyzed by 55Mn NMR spectroscopy. The difference of the 55Mn-signal from the blank and the water sample was recalculated with the concentration of the potassium permanganate solution into the reacted amount of potassium permanganate and thus into the consumption of oxygen.
What have we found?
The results of the water quality are presented in Tab.2. As expected, the signal of the 55Mn decreased with a higher amount of oxidizable substances in the water samples because the permanganate was reduced to another oxidation state which cannot be analyzed by 55Mn NMR spectroscopy – it “disappears” for the NMR spectrometer (Fig. 2).
Fig. 2 55Mn NMR spectra of collected water samples after reaction with potassium permanganate
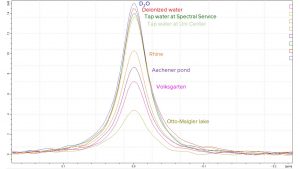
Tap water, which is one of the most inspected food products in Germany, had a very low concentration of oxidizable substances and, thus it was labelled as very clean. Very interesting was the fact that rivers showed a better water quality than lakes. The reason for that is quite simple: rivers are flowing waters and lakes are stagnant waters. The advantage of flowing waters is that with a higher flow rate the exchange of the water is high so that contaminations are only temporary present. Since the temperature of flowing waters is lower than in stagnant waters, bacteria, fungus and algae are less present here.
Tab. 2 Potassium permanganate consumption of the collected water samples
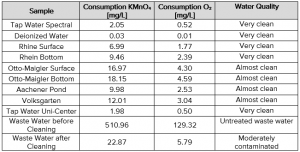
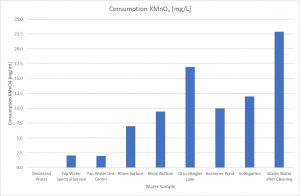
Another interesting result was the difference of the potassium permanganate index of the Rhine water, collected from the surface and the bottom of the river. As expected, the surface of the Rhine is cleaner than the bottom. One reason is also the flow rate which increases the exchange, and the other reason is that contaminations and soil are gradually to be deposited on the bottom. During the sample collection, these particles can whirl up influencing the water quality result.
Fig. 3 Potassium permanganate consumption of the collected water samples
Does it mean, that I can use the NMR spectroscopy to determine the quality of water?
Yes, of course! As presented, the 55Mn NMR spectroscopy is a very powerful tool for an alternative analysis of water quality concerning the quantity of organic matter. NMR spectroscopy is a fast and robust method with a great potential to replace the conventional titration method.